Abstract
Background: While incorporation of HLA-allele matching has highlighted high degrees of CB unit-recipient HLA-disparity, the less stringent HLA-match requirements extends allograft access to many patients without matched adult donors. The extent to which HLA-mismatch can adversely affect CBT outcomes, & the maximum permitted degree of HLA-mismatch, however, are not established.
Methods: We analyzed TRM, relapse & PFS after intermediate intensity myeloablative cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 5-10 mg/kg, 400 cGy TBI double unit CBT in adults transplanted for hematologic malignancies. Eligible patients (pts) were first allograft recipients ≤ 65 years transplanted for acute leukemia/ MDS/ MPD (≤ 10% blasts pre-CBT), B-cell or T-cell lymphomas between 1/2014 - 12/2017. TNC & CD34+ dose were typically given priority over HLA-match in unit selection.
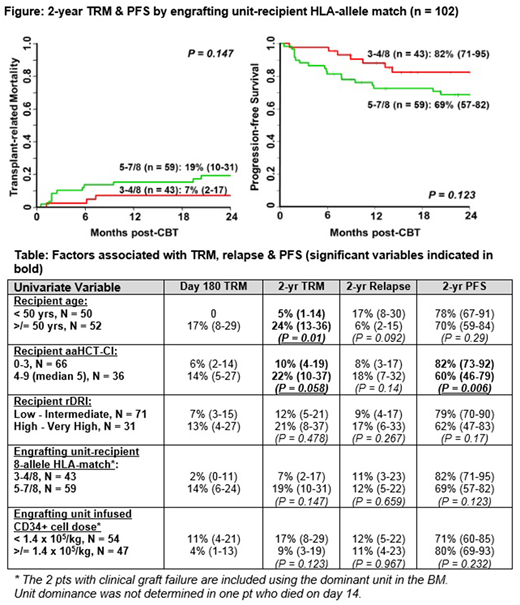
Results: 102 pts [51 (50%) non-European, 59 (58%) CMV seropositive, median age 50 years (range 21-65), median weight 80 kg (range 36-137)] were transplanted. Diagnoses included 71 acute leukemias, 17 CML/ MPD/ MDS, 14 B- or T-cell NHLs. All received double unit grafts comprised of 2 (1%) 6/6 units, 21 (10%) 5/6, 181 (89%) 4/6 HLA-match, median unit-recipient 8-allele HLA-match 5/8 (range 3-7), & median infused viable CD34+ dose 1.3 x 105/kg/unit (range 0.2-8.6). Forty-eight (47%) CB grafts were supplemented with haplo-identical CD34+ cells as a myeloid bridge. The cumulative incidence of sustained CB engraftment was 97% (95%CI 90-99) with 2 pts having graft failure. A dominant (engrafting) unit was identified in all pts but 1 who died on day +14. Day 180 incidences of grades II-IV & III-IV aGVHD were 89% (95%CI 81-94) & 23% (95%CI 15-31), respectively. 1-yr cGVHD was 4% (95%CI 1-9). Cumulative incidence of TRM was 9% (95%CI 4-15) at day 180 & 14% (95%CI 8-22) at 2 yrs. 11% (95%CI 6-19) of pts relapsed by 2 yrs. With a median survivor follow-up of 2 yrs 3 months (range 6 - 51), the 2-yr overall survival is 82% (95%CI 75-90) & PFS is 74% (95%CI 66-84). Causes of death were transplant-related in 16 pts [6 aGVHD, 4 infection (2 viral, 1 bacterial, 1 fungal), 4 organ failure, 1 graft failure, 1 unknown] & due to relapse in 4 pts. By 2-yrs post-CBT, engrafting unit-recipient HLA-allele match had no association with TRM or PFS (Figure). Univariate analysis of the association between recipient variables [age, age adjusted hematopoietic cell transplant-comorbidity index (aaHCT-CI), revised disease risk index (rDRI)] & graft variables [engrafting unit-recipient HLA-allele match & infused CD34+ cell dose] & TRM, relapse & PFS are shown (Table). Age & aaHCT-CI were associated with 2-yr TRM with the 50 younger pts (< 50 years), & the 66 pts with aaHCT-CI 0-3, having especially low 2-yr TRM of 5% & 10%, respectively. Neither engrafting unit-recipient HLA-match nor CD34+ dose were associated with TRM. No pt or graft variable was associated with relapse. The only significant variable associated with 2-yr PFS was aaHCT-CI. The 66 pts with a lower aaHCT-CI score of 0-3 had a higher 2-year PFS [82% (95%CI 73-92)] than the 36 high score 4-9 pts [60% (95%CI 46-79)]. rDRI, engrafting unit-recipient 8-allele HLA-match & CD34+ cell dose had no effect. Multivariate analysis of TRM was precluded by too few events. Multivariate analysis of PFS including aaHCT-CI, rDRI & engrafting unit-recipient HLA-match showed high aaHCT-CI was associated with worse PFS [HR 2.82 (1.28-6.25) if score 4-9 vs 0-3, p = 0.01]; neither rDRI [HR 1.21 (0.54-2.69) if high-very high vs low-intermediate, p = 0.64] nor engrafting unit-recipient HLA-match [HR 2.06 (0.89-4.74) if 5-7/8 vs 3-4/8 matched (p = 0.09] were significant.
Conclusions: Our finding that a high level of HLA-allele mismatch of the engrafting unit was not significantly associated with TRM or PFS in adult dCBT for hematologic malignancies has multiple implications. It supports the use of units with a relatively high degree of HLA-mismatch in the dCBT setting if needed in these pts. This facilitates extension of allograft access to the majority of pts including minorities. Moreover, the high PFS supports our centers unit selection strategy that gives unit quality & cell dose a priority in adults with malignancies to optimize engraftment. Finally, this data support CBT in many high-risk or urgent pts even if a well-matched CB graft cannot be identified. This is especially true in younger pts or those with a low 0-3 aaHCT-CI who had high 2-yr PFS.
Perales:Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Personal fees and Clinical trial support; Novartis: Other: Personal fees; Abbvie: Other: Personal fees; Merck: Other: Personal fees; Takeda: Other: Personal fees. Sauter:Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Precision Biosciences: Consultancy; Kite: Consultancy. Shah:Janssen: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal